Research
Our research interests fall under the broad scope of organic synthesis and include: total synthesis, structure determination, methods development, structure and reactivity of unstable species, and rearrangement chemistry. We are interested in: the development of key reaction methodology (especially in the areas of radical chemistry), transition metal catalysed processes and catalytic enantioselective synthesis; all aspects of the structure and reactivity of oxonium ions; and the use of biosynthesis and computational methods as predictive tools for the structure determination of complex molecules. All of the above inform and guide our research on complex molecule total synthesis.
The development of new synthetic methodology is a key focus of our research. We have a keen interest in transformations which result in a significant increase in molecular complexity and functionality from substrate to product, and have developed radical reactions for application in the synthesis of highly congested molecules and in the efficient synthesis of natural products as well as to the functionalisation of arenes. In recent work, we have developed a number of oxidative radical reactions and the sequencing of these radical reactions with subsequent ionic reactions allows the formation of complex molecular architectures from readily accessible substrates. Our investigations into transition metal catalysed and organocatalytic methodology has informed the stereocontrolled synthesis of complex, functionalised molecular building blocks for use in the synthesis of biologically relevant targets including natural products. Additionally, we are investigating the chemistry of oxonium ions for use in organic synthesis.
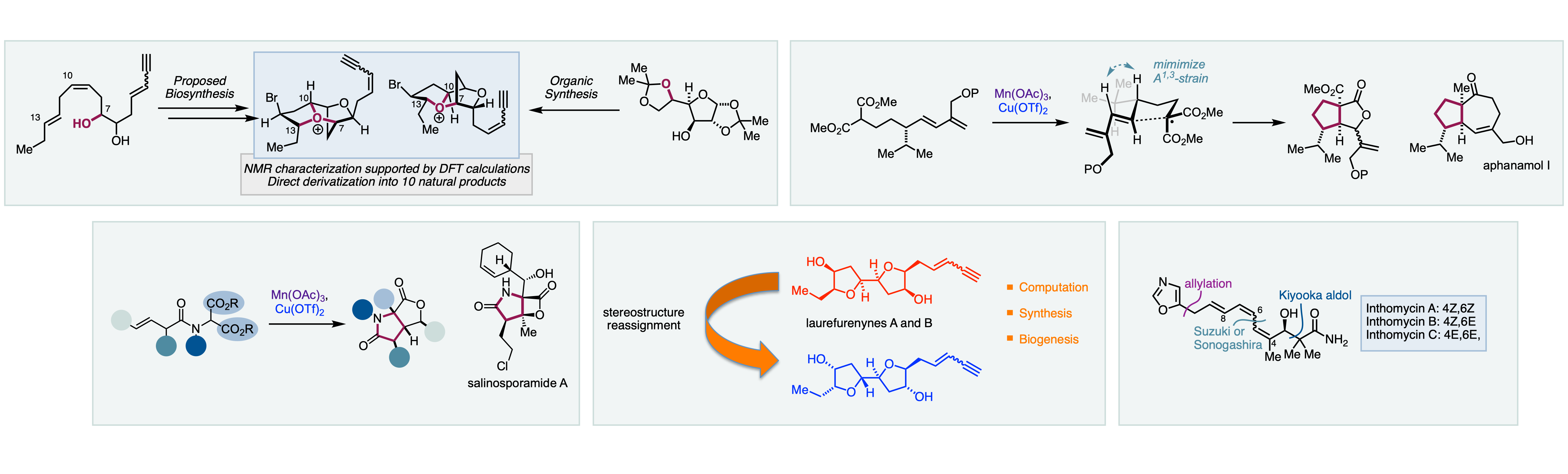
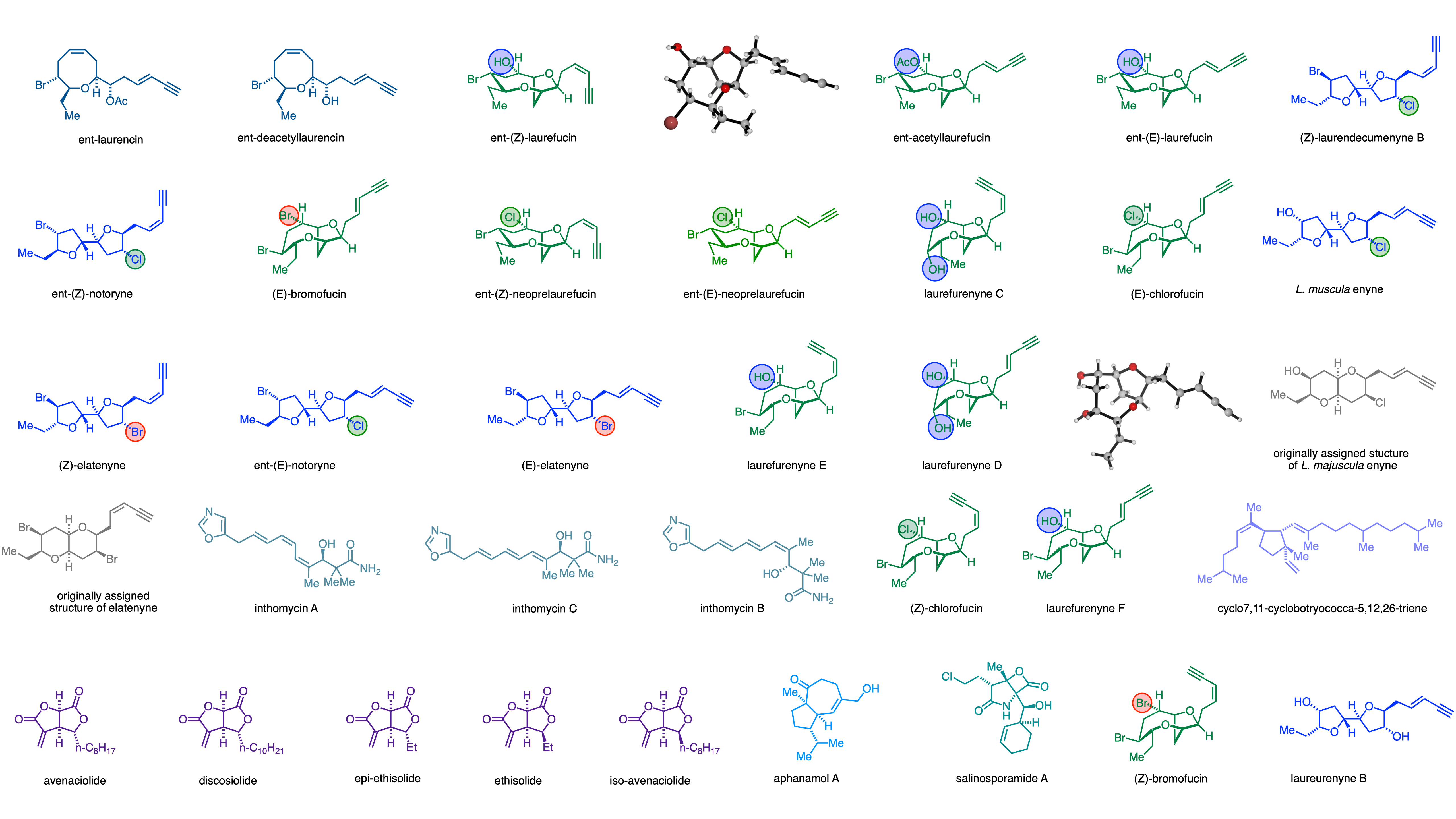
Natural products continue to provide an important source of chemical entities for the treatment of human disease. We use biologically active natural products as a source of inspiration for chemical methods development, to test our developed synthetic methodology and as important targets for total synthesis. A selection of current and completed targets are shown. We have applied our radical methodology to the total synthesis of a number of natural products including: 7,11-cyclobotryococca-5,12,26-triene, aphanamol A, the aveniciolides, ethisiolides, and discosiolides, as well as the potent proteasome inhibitor salinosporamide A. We have reported a modular route to the biologically active inthomycin natural products. Using our methodologies we have completed the total synthesis of more than fifteen natural products from Laurencia species using well characterised tricyclic oxonium ions as pivotal synthetic intermediates. Much of this work is enabled by thinking biomimetically and has been further facilitated by collaboration with computational chemists (Robert Paton). This powerful combination of computation and biosynthesis has led us to the structural reassignment of a number of natural products from Laurencia species.
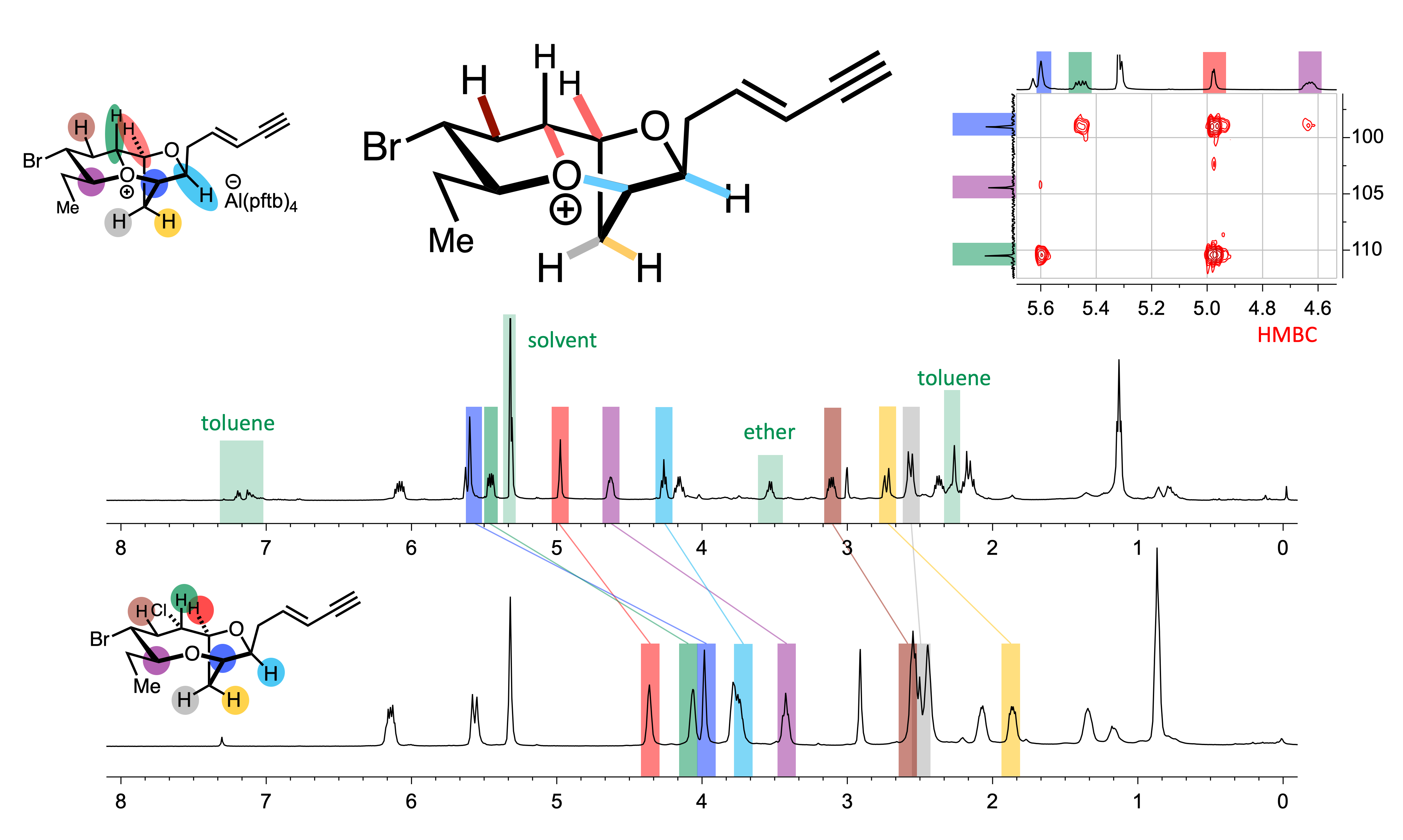
We have synthesised, characterised and studied the reactivity of a number of structurally and stereochemically complex tricyclic oxonium ions that have been proposed as key reactive intermediates in the biosynthesis of several natural products and we are further exploring biosynthetically related molecules. Currently we are investigating all aspects of the structure and reactivity of various classes of oxonium ions, which involves much novel and exciting chemistry.